 Obliquebanded Leafroller, Choristoneura
rosaceana
(Harris)
Obliquebanded Leafroller, Choristoneura
rosaceana
(Harris)
I. Introduction: The obliquebanded
leafroller
(OBLR) is native to and widely distributed throughout
temperate North America. Larvae feed on a wide range of plants
including unrelated deciduous trees
(e.g., larch). Their importance is increasing in the northeastern
U. S.
due to the development of
resistance to certain organophosphate insecticides. There are two
generations a year.
II. Hosts: Although the OBLR can feed on a wide range of
plants,
their preferred hosts are
members of the rose family. Outbreaks can occur on apple, peach,
nectarine, cherry and pear trees.
III. Description: Adults are approximately 3/4-1 inch
(19-25 mm)
long and have a wingspan of
1 inch (25 mm). Their forewings are reddish brown crossed by three
alternating light and brown bands
(Plate 26). The hind wings, invisible when the moth is at rest,
are
pale yellow. The female moth is
large and more distinctly marked than the male. A female can lay
up to
900 eggs during her 7- to 8-day
oviposition period. Eggs are laid on the upper surfaces of leaves.
They
appear as greenish, yellow masses
measuring about 3/16 x 3/8 inch (5 x 9.5 mm) and may contain 200
or
more eggs. The black head capsules
of embryonic larvae become visible prior to hatching, which
usually
occurs in 10 to 12 days. Larvae
are indiscriminate feeders that pass through six instars. Newly
hatched
larvae have a yellowish, green
body and a black head and thoracic shield. Mature larvae are
approximately 1 inch (25 mm) long, and
the head and thoracic shield may either be black or various shades
of
brown (Plate 27). These larvae
can be confused with larvae of the fruittree leafroller. Pupae are
dark
brown, about 1/2 inch (12.5 mm)
long, and are usually found in rolled leaves on the tree.
Occasionally
a species resembling obliquebanded leafroller occurs in pheromone
traps
of variegated leafroller. There is little overlap in the pheromone
blends of these species. The moths in the VLR traps are actually a
species related to obliquebanded leafroller, the spotted fireworm,
Choristoneura
parallela (Robinson). Spotted
fireworm
wings possess a dark spot on the leading edge, about a third
of the
length from the base, absent on obliquebanded
leafroller
wings.
IV. Biology: The OBLR overwinters as either second or third
stage larvae within a silken case,
or hibernaculum. These hibernacula can be found in the protected
areas
of the scaffold limbs (e.g.,
pruning scars). The larvae become active in the spring as the buds
begin to open. They begin to feed
by tying together a number of leaves with silk. Overwintered
larvae
feed first on watersprouts and then
move throughout the tree. Those feeding on developing flower buds
do so
before bloom and continue to
consume floral parts throughout the blossom period. After petal
fall,
these larvae continue to feed
on developing fruit. Pupation occurs within these sheltered areas
with
the first adults observed during
late May and early June. The first summer brood of larvae emerge
and
complete development in mid to
late July. Newly hatched larvae of the first summer brood move to
and
feed on tender growing terminals,
watersprouts, or developing fruit. As these larvae reach the third
instar they display an increasing
propensity to damage fruit. Second-brood eggs begin to hatch in
early
to mid-August and feed until
they reach the third instar in fall, when they construct
hibernation
sites on twigs or bark and enter
winter diapause. These second-brood larvae feed primarily on
leaves
until they enter diapause, although
they may occasionally damage fruit.

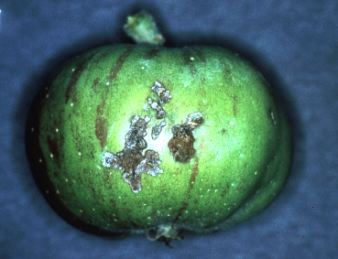
V. Injury: Larvae primarily feed on foliage, a habit which
has
no economic impact on mature
trees, but can seriously reduce leaf area and growth on nonbearing
trees. Feeding on the developing
fruit can occur at three times during the season. The first period
occurs at pink through the petal
fall and shortly thereafter. This feeding injury can cause fruit
to
abort or result in deeply scarred
fruit (Plate 28). The second feeding period occurs during July
when the
larvae can attach a leaf to
the surface of the fruit and feed. This injury is characterized by
shallow feeding usually of less
than 1/16 inch (1.6 mm) deep. More extensive injury may occur if
larger
larvae continue to feed on
the fruit. Feeding injury at this time of the season may become
scabbed
over by harvest. The third
feeding period occurs, just before, or during harvest. The type of
injury caused is similar to that
caused by the tufted apple bud moth, that of "pin-holing".
VI. Monitoring: The flight of the male adults can be
monitored
with sex pheromone traps. This
information can be used to predict when the various stages of OBLR
may
be present for timing sprays
(see below). However, since the males are capable of traveling
long
distances, sex pheromone traps
cannot be used to predict population size. Scientists at Cornell
University have developed a sequential
sampling plan for monitoring larval populations and determining
population size. Sequential sampling
utilizes the results of the samples that are being evaluated to
determine the total number of samples
to be taken. The following is taken from the Cornell 1993 Pest
Management Recommendations for Commercial
Tree-Fruit Production.
Figure 6 presents a sequential sampling chart for OBLR monitoring
at
bloom. Examine 10 bud clusters or
expanding terminals per tree for live larvae. Sample from random
trees
that are representative of the
entire block. Do not count actual numbers of larvae in an infested
cluster, damaged clusters that have
no larvae in them, or clusters containing only dead larvae. Choose
half
of your clusters or terminals
from the inside the tree canopy, and the other half from near the
outside of the canopy. As you proceed
from tree to tree, compare the number of clusters containing live
larvae with the limits given in the
figure. Continue sampling as long as you are between the two
values
given for the respective number
of samples taken; stop sampling when you reach one of these
values.
This could occur by the time 10
clusters have been sampled if 2 are infested, or after 60 clusters
have
been sampled and you remain
between the values. A result of "Stop Sampling and Treat" means
that an
appropriate insecticide should
be incorporated into the petal fall spray. "Stop Sampling, Don't
Treat"
means that the larval population
is not high enough and should not be considered when choosing
insecticides for the petal fall spray. If
you are at "3" by the 100th cluster, consider your infestation
level
below the threshold.
Figure 8 presents a sequential sampling chart for obliquebanded
leafroller monitoring for the first
summer generation. Sample at approximately 600 DD (43�F) after the
first adult flight in your
area. Traps should be hung by the end of May or early June
depending on
the locality. Examine
10 expanding leaf terminals per tree, selecting trees from as wide
an
area of the block as
possible. If trees are >3 m (>10 ft) tall, an effort should
be
made to include some clusters from
the mid- to upper canopy area, or from watersprouts, which are
favored
infestation sites. Try not
to bias your sample by picking clusters that you suspect are
infested.
Record the number of clusters
that are infested with live larvae, not the number of live larvae.
Continue sampling until you reach
one of the staircase lines in Figure 3. If you reach the
intersection
of the two lines by the 100th
cluster, this is equivalent to a Don't Treat decision. If you
reach a
Treat decision, an insecticide
spray is recommended at this time. If you reach a Don't Treat
decision,
return in 3-5 days (100 more DD)
and repeat the sample; a Don't Treat decision the second time
indicates
that no treatment is recommended.
Cornell University utilizes a 3% threshold at bloom (Figure 6) and
3
(Figure 6) and 10% (Figure 8) thresholds
for fresh and processing fruit, respectively, for the first summer
generation.
This is taken primarily from a chapter by L.
A.
Hull, D. G. Pfeiffer & D. J. Biddinger on apple direct pests,
reprinted with permission from Mid-Atlantic Orchard Monitoring
Guide,
published
by
NRAES, 152 Riley-Robb Hall, Ithaca, New York 14853-5701.
Back to Virginia
Apple
Page
Back to Home Page for "Arthropod Management
in
Fruit Crops" course
Back to Virginia
Fruit
Page
 Obliquebanded Leafroller, Choristoneura
rosaceana
(Harris)
Obliquebanded Leafroller, Choristoneura
rosaceana
(Harris)

